Recent Blog Posts
Is Joining a CPAP Class-Action Lawsuit Best for Me?
 More than five million people with sleep apnea have used CPAP machines hoping to get some rest. Unfortunately, many of them have ended up developing serious health problems. If you relied on a CPAP machine and you suffered illness or injury because of the device, you may be able to pursue compensation for your injuries from the manufacturer. It can be confusing to know if you should pursue a class-action lawsuit or file an individual claim. In this article, we will discuss the differences.
More than five million people with sleep apnea have used CPAP machines hoping to get some rest. Unfortunately, many of them have ended up developing serious health problems. If you relied on a CPAP machine and you suffered illness or injury because of the device, you may be able to pursue compensation for your injuries from the manufacturer. It can be confusing to know if you should pursue a class-action lawsuit or file an individual claim. In this article, we will discuss the differences.
Understanding What Triggered the Recall
Philips Respironics recalled CPAP machines that were manufactured from 2009 to 2021, because the polyurethane foam (PE-PUR) used to soundproof the machine exposed users to toxic particles while they inhaled.
Since the recall was announced in the summer of 2021, multiple class-action lawsuits have been filed against Philips. However, these lawsuits have been based on claims of consumer fraud rather than injuries suffered by users. These consumers were trying to seek reimbursement to obtain replacement devices to deal with their sleep apnea. People can benefit from a class action lawsuit if there is a large number of people impacted by the negligence of a single company. When a verdict or a settlement is reached, the damages awarded are distributed among those who joined the class-action lawsuit.
The Recall of Three CPAP Machines for Sleep Apnea
 Devices that are supposed to help people who suffer from sleep apnea have been harming users. Three types of continuous positive airway pressure or CPAP machines have been recalled to date. That is because millions of people who used these CPAP, BiPAP, or ventilator machines may have been exposed to toxic particles when they were breathing.
Devices that are supposed to help people who suffer from sleep apnea have been harming users. Three types of continuous positive airway pressure or CPAP machines have been recalled to date. That is because millions of people who used these CPAP, BiPAP, or ventilator machines may have been exposed to toxic particles when they were breathing.
The Recall
Philips Respironics has recalled CPAP machines manufactured between 2009 to June 2021, because their soundproofing foam degrades over time. CPAP machines are designed to keep airways open by providing a consistent stream of air through a mask so that people with sleep apnea can get a good night’s rest. But the polyurethane foam (PE-PUR) has exposed users to toxic particles. There is a danger of inhaling and choking on pieces of the foam. This remains an ongoing issue that has yet to be resolved.
Recalled Devices
Federal Authorities Investigating Increase in CPAP-Related Injuries
 The number of people impacted by a defective CPAP machine to combat their sleep apnea continues to grow. There have been more than 90,000 reports about problems with the devices, including 260 reports of people who died—all since April 2021. The FDA is now conducting an in-depth review into why these numbers have spiked in the last year and a half. Our attorneys understand that you expected your medical device to work properly and not cause more serious health problems. It is time for you to take action and get the compensation you deserve for your serious injuries.
The number of people impacted by a defective CPAP machine to combat their sleep apnea continues to grow. There have been more than 90,000 reports about problems with the devices, including 260 reports of people who died—all since April 2021. The FDA is now conducting an in-depth review into why these numbers have spiked in the last year and a half. Our attorneys understand that you expected your medical device to work properly and not cause more serious health problems. It is time for you to take action and get the compensation you deserve for your serious injuries.
Health Conditions Caused by Foam
Philips Respironics issued a recall last summer because the machines manufactured between 2009 to June 2021 were defective. The machines used soundproofing foam, but as the PE-PUR foam degraded over time, more than five million people who used these CPAP, BiPAP, or ventilator machines may have been exposed to harmful particles while breathing.
Forms of Compensation for CPAP Injuries
 As efforts to replace CPAP machines now stretch into next year, what can sleep apnea users do? More than five million people may have been exposed to toxic substances in their effort to get a good night’s rest. Injuries may have led to ongoing chronic health issues and disabilities affecting a person’s quality of life. If the therapy that was meant to give you sound sleep has turned your life upside down with a cancer diagnosis and ongoing medical treatments you are entitled to seek compensation for your CPAP injuries.
As efforts to replace CPAP machines now stretch into next year, what can sleep apnea users do? More than five million people may have been exposed to toxic substances in their effort to get a good night’s rest. Injuries may have led to ongoing chronic health issues and disabilities affecting a person’s quality of life. If the therapy that was meant to give you sound sleep has turned your life upside down with a cancer diagnosis and ongoing medical treatments you are entitled to seek compensation for your CPAP injuries.
Dangerous Foam
Royal Philips recalled the machines last year because the foam used to reduce the noise level of the motor breaks apart releasing toxic chemicals. The pieces of foam have been swallowed by people thereby causing throat, liver, lung, and kidney cancers, among others. Sleep apnea machines have also led to serious injuries and deaths.
What Cancers Do CPAP Machines Cause?
 If you are like nearly 30 million Americans who suffer from sleep apnea, all you wanted was to have a restful night. Instead, it may feel like a nightmare if you have been told you have cancer after using CPAP machines. If you have been impacted by these dangerous devices we are committed to helping you with this medical device disaster.
If you are like nearly 30 million Americans who suffer from sleep apnea, all you wanted was to have a restful night. Instead, it may feel like a nightmare if you have been told you have cancer after using CPAP machines. If you have been impacted by these dangerous devices we are committed to helping you with this medical device disaster.
Cancer Due to Foam Dangers
If you are anything like a typical defective CPAP machine victim, you relied on special devices such as CPAP, BiPAP, or ventilator machines to get uninterrupted sleep and be able to go to work and take care of your children. Instead, you woke up coughing phlegm and black specks after using CPAP machines.
Royal Philips announced in the summer of last year that millions of CPAP machines and mechanical ventilators had hidden flaws. The company issued a recall because the machines could expel particles and gasses. The polyester-based polyurethane (PE-PUR) foam used to reduce the noise of the motor had been disintegrating and releasing toxic chemicals and debris into the air hoses. Inhaling or swallowing pieces of the foam could cause various cancers, serious injuries, or deaths.
CPAP-Related Lung Conditions That Could Lead to Lung Cancer
Properly-functioning lungs are a crucial component of people's overall health. Everyone needs to be able to breathe properly and absorb oxygen into their bloodstream. Any health conditions that affect lung functions can be incredibly harmful, and they may not only impact a person's overall health, but they may put the person at risk of losing their life. To make matters even worse, a person who experiences serious lung illnesses or injuries may also be at a higher risk of contracting lung cancer in the future.
Lung conditions have become a significant concern for many people who have used CPAP machines and similar devices to treat sleep apnea. In 2021, millions of these devices were recalled by the medical device manufacturing company Philips Respironics. The recalled CPAP and BiPAP machines contained a synthetic foam used to dampen the sound of the motors used to force air into users' airways. This foam, which was composed of potentially toxic substances, could break down over months or years of daily use. The inhalation of small particles of foam and other chemicals released due to the breakdown of foam may have led to multiple types of serious lung conditions, and some of these could potentially lead to lung cancer.
Are Users of Defective CPAP Machines at Risk of Kidney Cancer?
 CPAP machines are a common treatment for sleep apnea, but some models have been recalled due to defects that may pose a health risk. The recalled devices used a synthetic foam composed of polyester and polyurethane that was meant to reduce the noise when a device was operating. Unfortunately, this foam, which is toxic to humans, could break down during a device's regular use. As air passed through a device and was forced into a user's lungs, it may have picked up particles of foam, which could then be inhaled or swallowed by the user. This could potentially lead to a variety of health issues, including an increased risk of kidney cancer.
CPAP machines are a common treatment for sleep apnea, but some models have been recalled due to defects that may pose a health risk. The recalled devices used a synthetic foam composed of polyester and polyurethane that was meant to reduce the noise when a device was operating. Unfortunately, this foam, which is toxic to humans, could break down during a device's regular use. As air passed through a device and was forced into a user's lungs, it may have picked up particles of foam, which could then be inhaled or swallowed by the user. This could potentially lead to a variety of health issues, including an increased risk of kidney cancer.
Symptoms of Kidney Cancer
The absorption of tiny foam particles into the bloodstream may affect multiple organs and bodily systems. Because the kidneys act as a filter for the blood, foam particles may have accumulated in the kidneys and affected the organ's tissues. This may have led tumors to form, affecting kidney functions. As tumors grow, they may also affect other bodily systems, including spreading to nearby lymph nodes, major blood vessels, or the adrenal gland. In the most serious cases, cancer may metastasize throughout the body, affecting distant lymph nodes or other organs.
Can Materials in Recalled CPAP Machines Cause Liver Cancer?
 Sleep apnea, in which a person periodically stops breathing while they are sleeping, can seriously affect the quality of a person's sleep and their overall health. While multiple forms of treatment are available to address this issue, the most effective treatment for most people is to use a CPAP machine. These devices force air into a person’s nose or mouth while they are sleeping, keeping their airways open and ensuring that they do not stop breathing. Unfortunately, many people have lost their access to this form of treatment due to a recall of CPAP machines by a major medical product manufacturer. Due to this recall, people may not only need to deal with the loss of treatment, but they may also have been placed at risk of injury due to toxic materials in the recalled devices. There are a number of injuries and illnesses that may have affected CPAP users, including liver cancer.
Sleep apnea, in which a person periodically stops breathing while they are sleeping, can seriously affect the quality of a person's sleep and their overall health. While multiple forms of treatment are available to address this issue, the most effective treatment for most people is to use a CPAP machine. These devices force air into a person’s nose or mouth while they are sleeping, keeping their airways open and ensuring that they do not stop breathing. Unfortunately, many people have lost their access to this form of treatment due to a recall of CPAP machines by a major medical product manufacturer. Due to this recall, people may not only need to deal with the loss of treatment, but they may also have been placed at risk of injury due to toxic materials in the recalled devices. There are a number of injuries and illnesses that may have affected CPAP users, including liver cancer.
Can Defective CPAP Machines Lead to Throat or Esophageal Cancer?
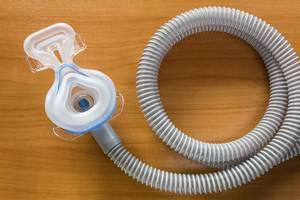 Many people use continuous positive airway pressure (CPAP) devices to treat sleep apnea or similar conditions. CPAP machines provide a steady stream of air that keep a person’s airways open, ensuring that they do not stop breathing while they are sleeping. These devices can provide numerous benefits, including helping people avoid the risks of heart conditions, ensuring that they get enough rest while sleeping, and improving their overall health.
Many people use continuous positive airway pressure (CPAP) devices to treat sleep apnea or similar conditions. CPAP machines provide a steady stream of air that keep a person’s airways open, ensuring that they do not stop breathing while they are sleeping. These devices can provide numerous benefits, including helping people avoid the risks of heart conditions, ensuring that they get enough rest while sleeping, and improving their overall health.
Unfortunately, a large number of CPAP users have learned that the products that were meant to benefit them may have put them at risk of injury. A massive recall of CPAP machines is currently being performed by Philips Respironics. The recalled devices contained a type of foam composed of polyester-based polyurethane, and the breakdown of this foam over time may have led foam particles and hazardous chemicals to be inhaled by users. These substances have been associated with multiple forms of cancer, including throat cancer and esophageal cancer.
U.S. Government Continues to Address the Philips CPAP Recall
 For nearly a year, millions of Americans who use CPAP devices to treat sleep apnea have been left up in the air due to a massive recall by Philips Respironics. The medical device manufacturing company began a voluntary recall of CPAP, BiPAP, and ventilator devices in June of 2021. However, because of the scale of the recall, millions of people have not yet been able to receive replacement devices, and many have been forced to choose between going without treatment or potentially putting themselves at risk of harm through the use of devices that may be unsafe. Multiple agencies in the United States government have been looking into the recall, and they may take action against Philips to address the ways consumers have been affected.
For nearly a year, millions of Americans who use CPAP devices to treat sleep apnea have been left up in the air due to a massive recall by Philips Respironics. The medical device manufacturing company began a voluntary recall of CPAP, BiPAP, and ventilator devices in June of 2021. However, because of the scale of the recall, millions of people have not yet been able to receive replacement devices, and many have been forced to choose between going without treatment or potentially putting themselves at risk of harm through the use of devices that may be unsafe. Multiple agencies in the United States government have been looking into the recall, and they may take action against Philips to address the ways consumers have been affected.
Actions Taken Against Philips by the FDA and DOJ
Philips began its recall of CPAP machines and similar devices because it learned that the products contained a dangerous substance that could break down over time. A polyester-polyurethane foam had been used in these devices to dampen sound and help them operate more quietly. Unfortunately, the breakdown of this foam may have led users of the devices to inhale tiny particles and toxic chemicals, leading to potential lung injuries and other health issues.
 20 N Clark Street, Suite 3300, Chicago, IL 60602
20 N Clark Street, Suite 3300, Chicago, IL 60602


